Breast Cancer is the most common cause of cancer death in women worldwide. Felix Lucka from the Computational Imaging group at CWI (the Dutch national research institute for mathematics and computer science) is part of a European research team that develops novel imaging techniques that will improve early detection and diagnosis in the future.
Between 1990 and 2019, the number of women diagnosed with breast cancer in the Netherlands has grown more than proportionally from 8300 to 17000 per year. Worldwide, breast cancer accounted for 25% of all cancer cases and 15% of all cancer deaths among women, making it the most common cancer diagnosis and cause of cancer death in women. While many advances in early detection and diagnosis have improved the survival chances of breast cancer patients considerably, there is urgent need for novel imaging techniques that provide higher specificity, contrast and image resolution than existing techniques like X-ray mammography and at lower costs than for example Magnetic Resonance Imaging (MRI).
Improved breast cancer imaging techniques
Two potential candidate imaging techniques to achieve this innovation are Photoacoustic Tomography (PAT) and Ultrasound Tomography (UST). Through its Horizon 2020 research and innovation program, the European Union funded the PAMMOTH project(1), in which a consortium of partners from academia, industry and the clinic from 6 different EU countries worked together to develop a combined PAT + UST scan device and validate its benefits for breast cancer diagnosis. Over more than four years, each partner brought in their individual expertise, ranging from applied physics, engineering, applied mathematics, computer science, ultrasound detection and laser technology to radiology. Felix Lucka joined the team while being a postdoc at University College London and continued his involvement after joining CWI.
In a series of blog posts,the contributions of applied mathematics and computer science to this exciting project are described. They can be can roughly summarized in three groups:
- Realistic in-silico modeling and simulation to support the hardware design phase of the project.
- Development and implementation of the image reconstruction algorithms that translate the photoacoustic and ultrasonic data measured by the scanner into diagnostic images.
- Validation and calibration of these algorithms with experimental data measured on test objects, volunteers and patients.
We start with the first topic and take a look at the modeling and simulation of PAT.
Light becomes sound
PAT relies on coupling optical and acoustic wave propagation processes by the photoacoustic effect: Firstly, the breast is illuminated by a very short pulse of laser light in the near-infrared spectrum, which does not cause harm to the patient (the wavelengths used in PAMMOTH range from 720nm to 870nm). The photons travel through the breast, get scattered and are eventually absorbed. The latter predominantly happens in regions with a high concentration of chromophores, for instance in the blood vessels which contain hemoglobin saturated or desaturated with oxygen (oxyhemoglobin vs. deoxyhemoglobin). The photoacoustic effect occurs when a sufficient part of the absorbed optical energy is converted to heat sufficiently fast and is not re-emitted: the induced, local pressure increase initiates acoustic waves traveling through the breast tissue on the microsecond timescale – light has become sound.
The out-going ultrasound waves can be measured by transducers that are acoustically coupled with the breast, for instance through a water bath. Image reconstruction methods then try to recover the spatial distribution of the absorbed optical energy from the ultrasonic measurements. This gives a high-resolution image of the blood vessels in the breast and the tumor. Obtaining this structural information can be used to detect and classify a tumor and is the first goal of the PAMMOTH project. The strength of absorption of each chromophore varies with wavelength in a unique way (absorption spectrum). If PAT images for multiple laser wavelengths are available, this spectral signature can be used to estimate local chromophore concentrations and through this, images of the blood oxygen saturation (the ratio of oxyhemoglobin vs. deoxyhemoglobin in blood). Obtaining this functional information is important for cancer diagnosis and is the second goal of the project.
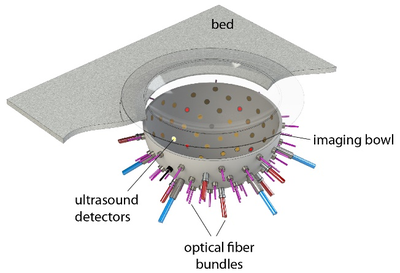
Figure 1: In the PAMMOTH scanner, the breast is supported by a plastic cup and placed in a bowl filled with water. Optical fibers and ultrasound transducers are installed in holes in the bowl.
To simulate the photoacoustic signal generation described above, first a model of the scanner including the positions and characteristics of the light sources and transducers (Fig 1) was constructed. A realistic digital phantom of a female breast that colleagues build for PAT and UST simulations(2) was placed in this scanner model. It consists of a map of the dominant breast tissues (Fig 2) derived from specific MRI measurements. Finally, the research team assigned realistic values for the optical and acoustic properties of the different tissues.
Based on a mathematical model of how photons travel within biological tissues, we could then estimate how the laser light spreads in the breast and where it is absorbed. This is done via extensive Monte Carlo simulations of the paths of individual photons that run on Graphical Processing Units (GPUs). The result of such a light transport simulation can then be translated to the local pressure increase that gives rise to the second part of the photoacoustic signal generation, the acoustic propagation. Mathematical models of how ultrasound waves propagate through biological tissues were translated to efficient computational code that can run on GPUs. Finally, an acoustic detection model simulates how the ultrasound waves are measured by the ultrasonic transducers used.
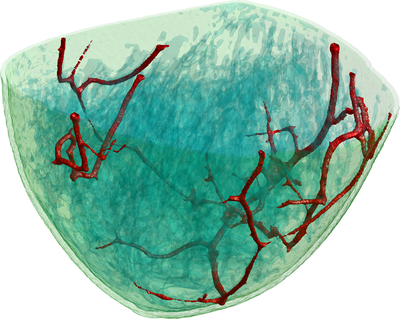
Figure 2: This illustration shows a volume rendering of the 3D digital breast phantom used in the simulation studies. The different colours represent different biological tissues.
Optimizing image quality
Simulating photoacoustic signals in this way was very useful in the early phase of the PAMMOTH project, in which the hardware of the scanner had to be decided. Some of these system design choices involved trading off different desirable properties. Unfortunately, it was often not a-priori clear which available option would lead to the best image quality and deciding it based on experiments would have costed too much time and resources. For example, the researchers had to choose between different possible ultrasound transducer designs each with advantages and disadvantages but building and testing all of them would have not been feasible. Instead, a simulation was made of how each of them would influence the photoacoustic signal and how that would impact the quality of the reconstructed PAT images (Fig 3). Using such in-silico experiments allowed the team to make well-informed design choices that optimized the image quality of the scanner before a single physical part of it was even build.
In the following blog, Felix Lucka will describe the process of photoacoustic image reconstruction in more detail, namely how mathematical algorithms translate the recorded ultrasonic measurements into images.
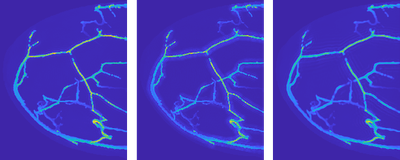
Figure 3: Visualization of simulated PAT reconstructions using three different ultrasound transducer models. Left: The idealized but practically unachievable design gives an idea of the best-case image quality. Middle and right: Two different realistic designs each lead to different image imperfections, visible as shadow-like artifacts around the blood vessels.
References
(1) “Photoacoustic/Ultrasound Mammoscopy for evaluating screening-detected lesions in the breast”. This project has received funding from the European Union's Horizon 2020 research and innovation program H2020 ICT 2016-2017 under grant agreement No 732411.
(2) Lou, Zhou, Matthews, Appleton, Anastasio. Generation of anatomically realistic numerical phantoms for photoacoustic and ultrasonic breast imaging. Journal of Biomedical Optics, 22(4):041015, 2017.
About the author

This blog was written by Felix Lucka, who is a tenure track researcher in the Computational Imaging Group at CWI. More information on his research interests and his contact details can be found on his webpage
