During embryonic development, cells do not only communicate through chemical signals, but also through mechanical forces between the cells. Researchers of CWI and Cornell University in Ithaca (USA) have successfully reproduced the growth of a blood vessel structure solely from mechanical forces. Their research was published in PLoS Computational Biology last Thursday. This research contributes to a better understanding of embryonal development.
The development of an embryo from a single cell to a complex organism is still not fully understood. Most research focuses on the differentiation of cells in organs and tissues through chemical communications. Recent experiments have revealed the key role of mechanical forces. By pushing and pulling the extracellular matrix in which the cells are embedded, individual cells affect the movement and oriëntation of their neighbours.
Computational biologists of CWI have now developed a computer model that supports the key role of these mechanical forces during embryonal development. Their model simulates the growth of a blood vessel structure on a cellular level solely based on mechanical forces between cells. The simulations agree well with experiments on cultures of blood vessel structures performed at Cornell University.
The simulations provide a powerful new tool for modelling mechanical interactions between cells, and will contribute to a better understanding of embryonal growth and development of complex organisms, including humans.
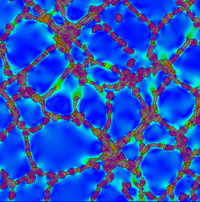
Image: Simulation of a vascular network generated by mechanical cell-cell communication. By pulling on a substrate, cells are able to generate strains that guide them to collectively form vascular-like netwokr. Colors on the substrate indicate strain magnitude, black lines represent the orientation of the highest strain. Cells are colored transparant red, such that strains underneath the cells are visible.
More information:
